Next to Alpha and Beta Kappa is one of the most commonly used Greek letters in university sorority and fraternity names. Ill type it here and hope Brainly can handle it.
Physics 12 Mr Jean January 13th Ppt Video Online Download
An alpha radioactive decay is the type of decay in which an atomic nucleus gives off an helium nucleus and is thereby transformed into another element which has a.

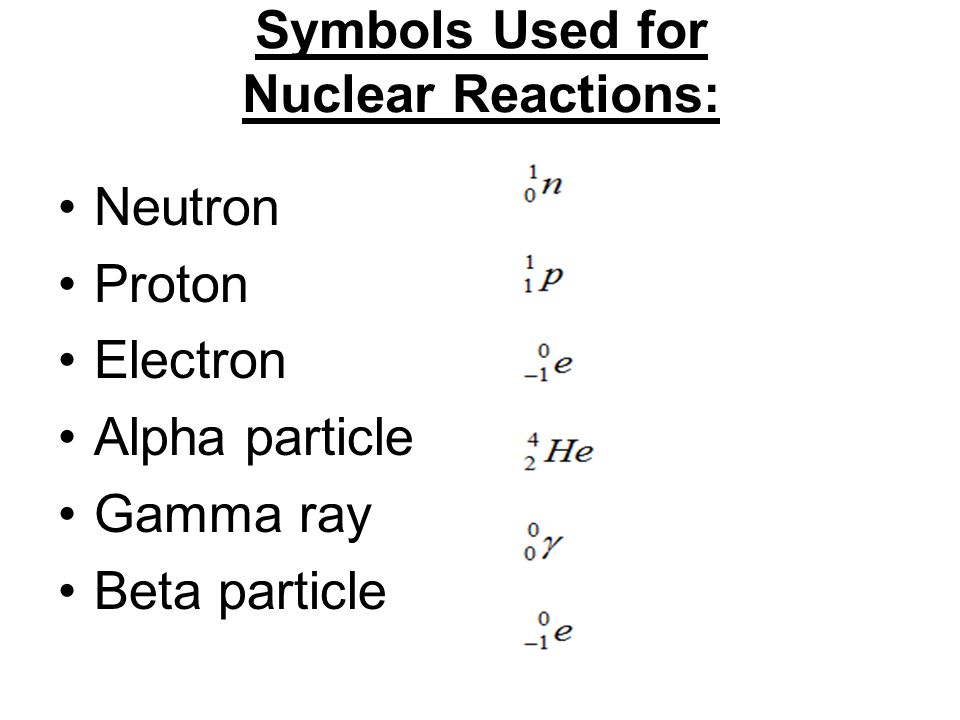
. The notation that is used to represent an alpha particle is 42 H THAT IS AN HELIUM ATOM. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. 37273 2 4 -1 gamma has no mass or charge.
Small Greek Alpha α. But since it is also a helium nuclei it can. Identify the particle represented by each symbol as an alpha particle a bet 0030.
Alpha particle mass number. An alpha particle also written as α-particle consists of 2 protons and 2 neutrons. Answered by Alexandra M.
Alpha particles are particles that consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleusThey are generally produced in the process of alpha decay but may also be produced in other ways. It is also 24He2 Symbol and charge of alpha particle. Called also alpha alpha ray.
What speed does an alpha particle which has been emitted travel at. Describe nuclear symbol notation. Do not write the symbol as a but in terms of a specific atom on the periodic table Click in the answer box activate the palette.
What is the symbol for an alpha particle. Chemistry questions and answers. Discovered and named 1899 by Ernest Rutherford alpha particles were used by him and.
For chemists Kappa is the symbol to represent the amount of oxygen required to bleach pulp. Alpha particles also called alpha rays or alpha radiation consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleusThey are generally produced in the process of alpha decay but may also be produced in other waysAlpha particles are named after the first letter in the Greek alphabet αThe symbol for the alpha particle is α or α 2. It transforms or decays into an atom with an atomic number 2 less and a mass number 4 less.
_88226Ra _86222Rn _24He Note that the sum of the subscripts atomic numbers or charges is. Describe the structure of an alpha particle. 664465723082 10-27 Kg.
Why can alpha be dangerous to the body. The nuclear symbol for an alpha particle is The name for the greek letter γ is fill in the blank 2. The uppercase letter alpha is A and the lowercase letter alpha is α.
Describe the relative penetrating powers of alpha beta and gamma radiation Add To Playlist Add to Existing Playlist. It is the same as the nucleus of a helium atom. The charge of an alpha particle is 2 and the symbol is 2.
An alpha particle is made up of two neutrons and two protons. Mass of alpha particle in amu. 10 the speed of light.
Strictly speaking an alpha particle is represented by the first letter of the Greek alphabet alpha. A positively charged nuclear particle identical with the nucleus of a helium atom that consists of two protons and two neutrons and is ejected at high speed in certain radioactive transformations. Write the symbols used to denote alpha beta and gamma radiation and give their mass and their charge.
Write the nuclear symbol for an alpha particle. Thus radium-226 decays through α-particle emission to form radon-222 according to the equation. Describe the relative penetrating powers of alpha beta and gamma radiation 0252.
Are alpha particles energetic. Alpha particles a are composite particles consisting of two protons and two neutrons tightly bound together Figure 1They are emitted from the nucleus of some radionuclides during a form of radioactive decay called alpha-decayAn alpha-particle is identical to the nucleus of a normal atomic mass four helium atom ie. The alpha particle is the _____ of all particles emitted by radioactive nuclei.
Alpha particle is formed when a radioactive element undergoes an alpha radioactive decay. What are alpha particles. Mass of alpha particle in eV.
Kappa is the tenth number of the Greek alphabet. Mass of alpha particle compared to proton. Kappa Κ κ.
Medical Definition of alpha particle. During α-decay an atomic nucleus emits an alpha particle. Alpha particles are relatively large and carry a double positive charge.
In the Greek numeral system the letter was taken as the symbol of 1 one. Etymologically alpha came from aleph the first letter of the Hebrew alphabet meaning ox in Phoenician. An alpha particle is the same thing as the nucleus of a helium atom.
Alpha particle positively charged particle identical to the nucleus of the helium-4 atom spontaneously emitted by some radioactive substances consisting of two protons and two neutrons bound together thus having a mass of four units and a positive charge of two. Alpha Particle mass in Kg. The production of alpha particles is termed alpha decay.
Write the nuclear symbol for an alpha particle. Its numerical value is 20. Create a New Plyalist.
What drops by 4 when an alpha particle is emitted. What are the Properties of an Alpha Particle. It presents the firstthe beginning.
An alpha particle has by far the most mass of the. The mass and charge of an alpha particle The ability to describe the situation in which alpha particles are referred to as helium nucleus Symbols used to represent alpha particles. The mass number of the emitting nucleus.
There is only one alpha particle and that is the helium-4 nucleus. Alpha particles are energetic nuclei of helium. Also because an alpha particle which is 2 protons and 2 neutrons is the nucleus of a helium-4 atom you may see He2 or 42He2 used to write symbolize an alpha particle.
Note that only the nucleus is given out. The symbol of the alpha particle is the Greek letter alpha which is. An alpha particle has a positive charge because the 2 protons have a positive charge.
α alpha 2 He 2. Usually just the lower-case Greek letter alpha. They are not very penetrating and a piece of paper.
A doubly ionised helium atom. Of the radiations alpha beta and gamma fill in the blank 3 is the most penetrating and fill in the blank 4 is the least penetrating.

5 3 Types Of Radiation Chemistry Libretexts

Alpha Symbol A Meaning Copy Paste Alpha And Omega

Alpha Decay Beta Decay Gamma Decay Electron Capture Positron Production Nuclear Chemistry Youtube
0 Comments